Photoelectron Spectroscopy: Illuminating the World of Molecules
Photoelectron spectroscopy, a cornerstone in the realm of analytical chemistry, plays a pivotal role in unraveling the mysteries of molecular structures and compositions. Let’s embark on a journey through the fascinating world of photoelectron spectroscopy, exploring its origins, principles, applications, and the latest technological advancements that have transformed this field.
1. Introduction
Defining Photoelectron Spectroscopy
Photoelectron spectroscopy, commonly known as PES, is a powerful analytical technique used to investigate the electronic structure of atoms and molecules. By examining the energy distribution of photoelectrons emitted when a material is exposed to photons, scientists gain valuable insights into the composition and arrangement of its electrons.
Importance in Analytical Chemistry
In the vast landscape of analytical chemistry, photoelectron spectroscopy stands out as an indispensable tool. Its ability to provide detailed information about the electronic states of materials makes it an invaluable asset in fields ranging from surface analysis to material science.
2. Historical Background
Early Developments
The roots of photoelectron spectroscopy trace back to the early 20th century when scientists began unraveling the mysteries of light-matter interactions. Pioneers like Albert Einstein and Max Planck laid the groundwork for understanding the photoelectric effect, a phenomenon crucial to the principles of PES.
Milestones in Photoelectron Spectroscopy
The journey of photoelectron spectroscopy has witnessed remarkable milestones, from the first observations of photoelectric emissions to the development of advanced spectrometers with unprecedented precision. These breakthroughs have propelled the technique to the forefront of modern analytical methods.
3. Principles of Photoelectron Spectroscopy
Interaction of Light with Matter
At the heart of photoelectron spectroscopy lies the interaction between light and matter. When photons strike a material, they can liberate electrons, creating a cascade of events that are meticulously studied to unveil the material’s electronic structure.
Photoelectric Effect
Central to photoelectron spectroscopy is the photoelectric effect, where photons incident on a material surface release electrons. The energy distribution of these emitted electrons serves as a fingerprint, offering vital clues about the material’s composition.
4. Types of Photoelectron Spectroscopy
X-ray Photoelectron Spectroscopy (XPS)
XPS, also known as ESCA (Electron Spectroscopy for Chemical Analysis), utilizes X-rays to excite electrons and provides detailed information about the elemental composition of a sample’s surface.
Ultraviolet Photoelectron Spectroscopy (UPS)
UPS, employing ultraviolet light, focuses on the analysis of valence electrons, shedding light on the molecular orbitals and bonding characteristics of a material.
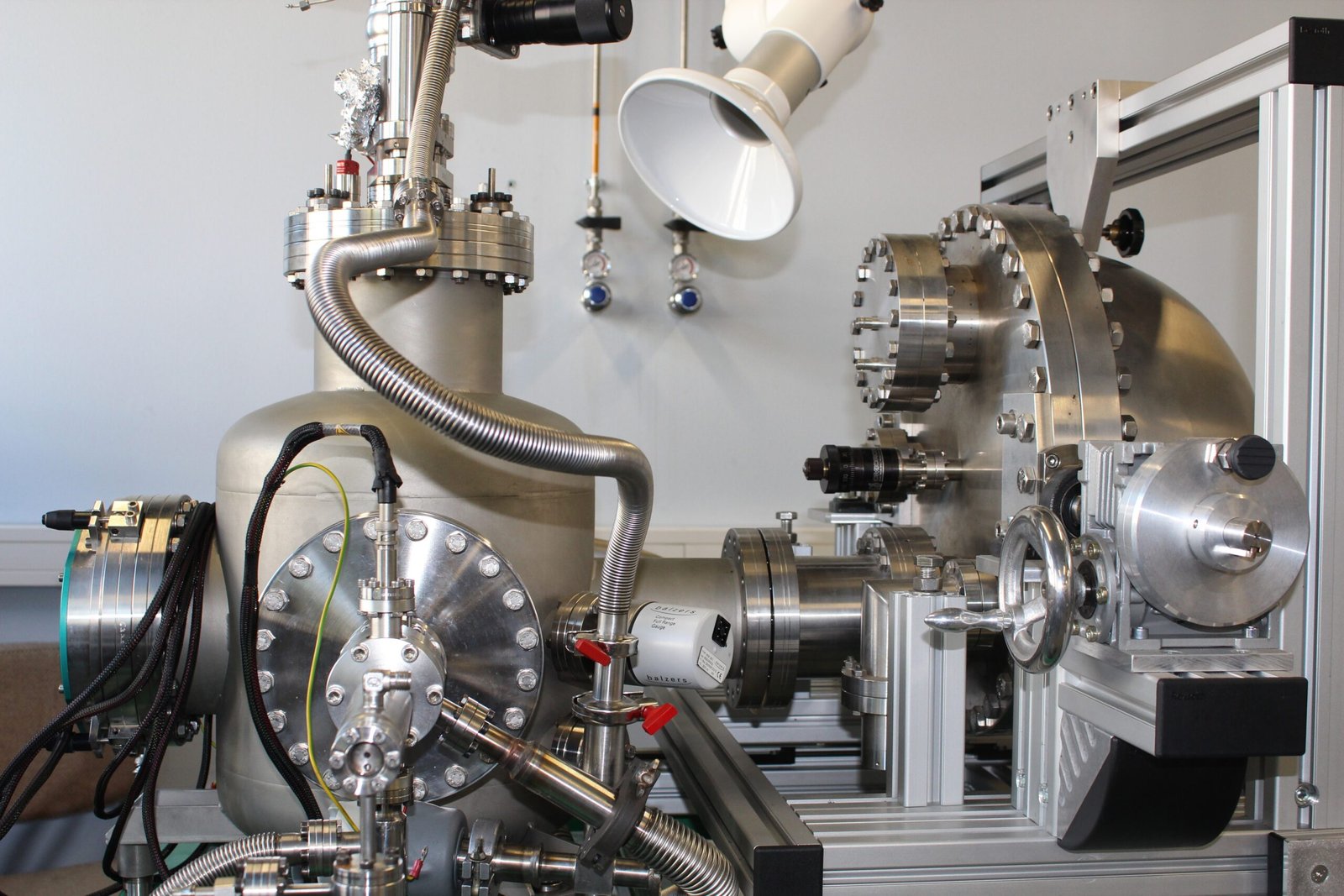
5. Instrumentation
Components of a Photoelectron Spectrometer
A photoelectron spectrometer comprises several critical components, including a photon source, an interaction region, and a detector. These components work in tandem to capture and analyze emitted photoelectrons.
Detector Systems
The efficiency and precision of a photoelectron spectrometer heavily rely on sophisticated detector systems capable of capturing and quantifying the emitted electrons.
6. Applications
Surface Analysis
One of the primary applications of photoelectron spectroscopy is surface analysis, where it provides detailed insights into the composition and structure of a material’s outermost layers.
Chemical Composition Identification
PES serves as a powerful tool for identifying the chemical composition of a substance, aiding researchers in characterizing unknown samples.
7. Advantages of Photoelectron Spectroscopy
High Sensitivity
The high sensitivity of PES allows researchers to detect trace amounts of elements, making it an invaluable technique in fields where precision is paramount.
Non-destructive Nature
The non-destructive nature of photoelectron spectroscopy ensures that the integrity of the sample is preserved, making it suitable for analyzing delicate materials.
8. Challenges and Limitations
Sample Requirements
Despite its many merits, PES does pose challenges related to sample requirements, often necessitating specific conditions for accurate results.
Sensitivity to Surface Conditions
The technique’s sensitivity to surface conditions can be a limitation, requiring meticulous sample preparation to achieve reliable outcomes.
9. Recent Technological Advances
Improved Resolution
Recent technological advancements have led to enhanced resolution in photoelectron spectroscopy, enabling researchers to explore electronic structures with unprecedented detail.
Time-of-Flight Spectroscopy
The integration of time-of-flight spectroscopy has further expanded the capabilities of PES, offering new insights into the dynamics of photoelectron emissions.
10. Future Trends
Emerging Applications
As technology advances, new applications for photoelectron spectroscopy continue to emerge, promising exciting possibilities in fields beyond traditional analytical chemistry.
Integration with Other Analytical Techniques
The future holds promise for the integration of PES with other analytical techniques, creating synergies that enhance the overall understanding of materials.
11. Case Studies
Real-world Examples of Photoelectron Spectroscopy Applications
Explore real-world case studies where photoelectron spectroscopy has played a pivotal role in solving scientific puzzles and advancing our understanding of diverse materials.
12. Tips for Successful Photoelectron Spectroscopy Experiments
Sample Preparation
Learn about the critical steps in sample preparation that can significantly impact the success of photoelectron spectroscopy experiments.
Data Interpretation
Master the art of interpreting PES data, turning complex spectra into meaningful information about the electronic structure of materials.
13. Importance in Material Science
Contributions to Material Characterization
Delve into the contributions of photoelectron spectroscopy to material science, shaping our ability to characterize and engineer novel materials.
14. Conclusion
In conclusion, photoelectron spectroscopy stands as a beacon in the world of analytical chemistry, unraveling the secrets of electronic structures with precision and finesse. From its historical roots to the latest technological innovations, PES continues to shape the landscape of scientific discovery.
