The Electron Configuration of Phosphorus: Unveiling its Atomic Structure
Introduction
The electron configuration of an element is a fundamental aspect of its atomic structure, describing the distribution of electrons in its orbitals. In this comprehensive exploration, we delve into the electron configuration of phosphorus (P), a crucial element found in biological molecules, fertilizers, and various industrial applications. Understanding the electron arrangement of phosphorus provides insights into its chemical behavior and reactivity. In this 1000-word essay, we will unravel the electron configuration of phosphorus, highlighting its significance in the periodic table and the implications for its chemical properties.
The Periodic Table and Phosphorus
Phosphorus is situated in the periodic table in the 15th group, also known as Group 15 or the Nitrogen Group. This group is characterized by elements that possess five valence electrons in their outermost electron shell. Phosphorus, with an atomic number of 15, shares similarities with nitrogen (atomic number 7), as both elements exhibit a valence electron configuration of ns²np³.
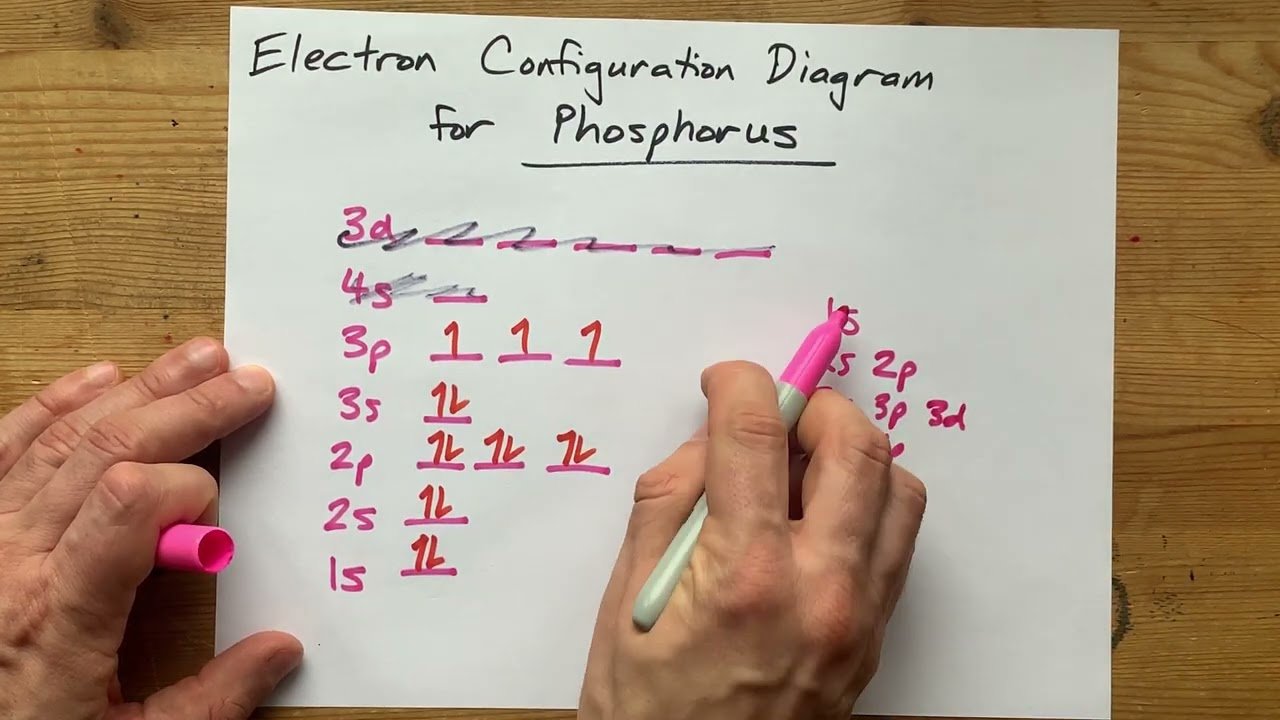
Atomic Structure of Phosphorus
The atomic structure of phosphorus consists of 15 electrons orbiting its nucleus. To understand its electron configuration, we need to fill its electron orbitals following the Aufbau principle, which states that electrons fill the lowest energy orbitals first. Phosphorus has three electron shells: the first shell (n=1) can hold up to 2 electrons, the second shell (n=2) can hold up to 8 electrons, and the third shell (n=3) can.
